Affordable-Water-Ionizers.com Is Affordable Nutrition For All |

Understanding The Science of Alkaline Water
By Dr. Hidemitsu Hayashi, M.D.
Heart Specialist and Director at the Water Institute of Japan
Nisshin Building, 2-5-10 Shinjiku,
Shinjiku-ku, Tokyo, Japan 160
Oxygen: Too much of a good thing?
Antioxidants block dangerous oxidation
How we can avoid illness
Water, the natural solution to avoid illness
What is Alkaline Ionized Water?
THE ALKALINE WATER IONIZER
Tap water: What it is and isn't
How an Alkaline Water Ionizer Works
What the Alkaline Water Ionizer Produces
Redox potential comparison
Redox potential, not pH, is the crucial factor
USING THE ALKALINE WATER IONIZER
What Alkaline Ionized Water Does
Reduced Water
Oxidized Water
Ionized Alkaline Water The Superior Antioxidant
SUMMARY AND CONCLUSIONS
Upstream and Downstream Theory
Prevent disease at the source
Upstream
Downstream
The water boom
Whenever we attempt to determine whether there is life as we know it on Mars or other planets, scientists first seek to establish whether or not water is present. Why? Because life on earth totally depends on water.
A High percentage of living things, both plant and animal are found in water. All life on earth is thought to have arisen from water. The bodies of all living organisms are composed largely of water. About 70 to 90 percent of all organic matter is water.
The chemical reactions in all plants and animals that support life take place in a water medium
. Water not only provides the medium to make these life sustaining reactions possible, but water itself is often an important reactant or product of these reactions. In short, the chemistry of life is water chemistry.
Water is a universal, superb solvent due to the marked polarity of the water molecule and its tendency to form hydrogen bonds with other molecules. One water molecule, expressed with the chemical symbol H2O, consists of 2 hydrogen atoms and 1 oxygen atom.
Standing alone, the hydrogen atom contains one positive proton at its core with one negative electron revolving around it in a three-dimensional shell. Oxygen, on the other hand, contains 8 protons in its nucleus with 8 electrons revolving around it. This is often shown in chemical notation as the letter O surrounded by eight dots representing 4 sets of paired electrons.
The single hydrogen electron and the 8 electrons of oxygen are the key to the chemistry of life because this is where hydrogen and oxygen atoms combine to form a water molecule, or split to form ions.
Hydrogen tends to ionize by losing its single electron and form single H+ ions, which are simply isolated protons since the hydrogen atom contains no neutrons. A hydrogen bond occurs when the electron of a single hydrogen atom is shared with another electronegative atom such as oxygen that lacks an electron.
In a water molecule, two hydrogen atoms are covalently bonded to the oxygen atom. But because the oxygen atom is larger than the hydrogen's, its attraction for the hydrogen's electrons is correspondingly greater so the electrons are drawn closer into the shell of the larger oxygen atom and away from the hydrogen shells. This means that although the water molecule as a whole is stable, the greater mass of the oxygen nucleus tends to draw in all the electrons in the molecule including the shared hydrogen electrons giving the oxygen portion of the molecule a slight electronegative charge.
The shells of the hydrogen atoms, because their electrons are closer to the oxygen, take on a small electropositive charge. This means water molecules have a tendency to form weak bonds with water molecules because the oxygen end of the molecule is negative and the hydrogen ends are positive.
A hydrogen atom, while remaining covalently bonded to the oxygen of its own molecule, can form a weak bond with the oxygen of another molecule. Similarly, the oxygen end of a molecule can form a weak attachment with the hydrogen ends of other molecules. Because water molecules have this polarity, water is a continuous chemical entity.
These weak bonds play a crucial role in stabilizing the shape of many of the large molecules found in living matter. Because these bonds are weak, they are readily broken and re-formed during normal physiological reactions. The disassembly and re-arrangement of such weak bonds is in essence the chemistry of life.
To illustrate water's ability to break down other substances, consider the simple example of putting a small amount of table salt in a glass of tap water. With dry salt (NaCl) the attraction between the electropositive sodium (Na+) and electronegative chlorine (Cl-) atoms of salt is very strong until it is placed in water. After salt is placed in water, the attraction of the electronegative oxygen of the water molecule for the positively charged sodium ions, and the similar attraction of the electropositive hydrogen ends of the water molecule for the negatively charged chloride ions, are greater than the mutual attraction between the outnumbered Na+ and Cl- ions. In water the ionic bonds of the sodium chloride molecule are broken easily because of the competitive action of the numerous water molecules.
|
|
|
As we can see from this simple example, even the delicate configuration of individual water molecules enables them to break relatively stronger bonds by converging on them. This is why we call water the universal solvent. It is a natural solution that breaks the bonds of larger, more complex molecules. This is the chemistry of life on earth, in water and on land.
Basically, reduction means the addition of an electron (e-), and its converse, oxidation means the removal of an electron. The addition of an electron, reduction, stores energy in the reduced compound. The removal of an electron, oxidation, liberates energy from the oxidized compound. Whenever one substance is reduced, another is oxidized.
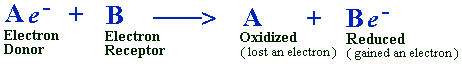
To clarify these terms, consider any two molecules, A and B, for example.
When molecules A and B come into contact, here is what happens:
B grabs an electron from molecule A.
Molecule A has been oxidized because it has lost an electron.
The net charge of B has been reduced because it has gained a negative electron (e-).
In biological systems, removal or addition of an electron constitutes the most frequent mechanism of oxidation-reduction reactions. These oxidation-reduction reactions are frequently called redox reactions.
An acid is a substance that increases the concentration of hydrogen ions (H+) in water. A base is a substance that decreases the concentration of hydrogen ions, in other words, increasing the concentration of hydroxide ions OH-.
The degree of acidity or alkalinity of a solution is measured in terms of a value known as pH, which is the negative logarithm of the concentration of hydrogen ions:
pH = 1/log[H+] = -log[H+]
On the pH scale, which ranges from 0 on the acidic end to 14 on the alkaline end, a solution is neutral if its pH is 7. At pH 7, water contains equal concentrations of H+ and OH- ions. Substances with a pH less than 7 are acidic because they contain a higher concentration of H+ ions. Substances with a pH higher than 7 are alkaline because they contain a higher concentration of OH- than H+. The pH scale is a log scale so a change of one pH unit means a tenfold change in the concentration of hydrogen ions.
Living things are extremely sensitive to pH and function best (with certain exceptions, such as certain portions of the digestive tract) when solutions are nearly neutral. Most interior living matter (excluding the cell nucleus) has a pH of about 6.8.

Blood plasma and other fluids that surround the cells in the body have a pH of 7.2 to 7.3. Numerous special mechanisms aid in stabilizing these fluids so that cells will not be subject to appreciable fluctuations in pH. Substances which serve as mechanisms to stabilize pH are called buffers. Buffers have the capacity to bond ions and remove them from solution whenever their concentration begins to rise. Conversely, buffers can release ions whenever their concentration begins to fall. Buffers thus help to minimize the fluctuations in pH. This is an important function because many biochemical reactions normally occurring in living organisms either release or use up ions.
NOTE: Dr. Hayashi is a Heart Specialist and Director of the Water Institute of Japan.
Oxygen: Too much of a good thing?
Oxygen is essential to survival. It is relatively stable in the air, but when too much is absorbed into the body it can become active and unstable and has a tendency to attach itself to any biological molecule, including molecules of healthy cells. The chemical activity of these free radicals is due to one or more pairs of unpaired electrons.
About 2% of the oxygen we normally breathe becomes active oxygen, and this amount increases to approximately 20% with aerobic exercise.
Such free radicals with unpaired electrons are unstable and have a high oxidation potential, which means they are capable of stealing electrons from other cells. This chemical mechanism is very useful in disinfectants such as hydrogen peroxide and ozone which can be used to sterilize wounds or medical instruments. Inside the body these free radicals are of great benefit due to their ability to attack and eliminate bacteria, viruses and other waste products.
Active Oxygen in the body
Problems arise, however, when too many of these free radicals are turned loose in the body where they can also damage normal tissue.
Putrefaction sets in when microbes in the air invade the proteins, peptides, and amino acids of eggs, fish and meat. The result is an array of unpleasant substances such as:
Hydrogen sulfide
Ammonia
Histamines
Indoles
Phenols
Scatoles
These substances are also produced naturally in the digestive tract when we digest food, resulting in the unpleasant odor evidenced in feces. Putrefaction of spoiled food is caused by microbes in the air; this natural process is duplicated in the digestive tract by intestinal microbes. All these waste products of digestion are pathogenic, that is, they can cause disease in the body.
Hydrogen sulfide and ammonia are tissue toxins that can damage the liver. Histamines contribute to allergic disorders such as atopic dermatitis, urticaria (hives) and asthma. Indoles and phenols are considered carcinogenic. Because waste products such as hydrogen sulfide, ammonia, histamines, phenols and indoles are toxic, the body's defense mechanisms try to eliminate them by releasing neutrophils (a type of leukocyte, or white corpuscle). These neutrophils produce active oxygen, oddball oxygen molecules that are capable of scavenging disintegrating tissues by gathering electrons from the molecules of toxic cells.
Problems arise, however, when too many of these active oxygen molecules, or free radicals, are produced in the body. They are extremely reactive and can also attach themselves to normal, healthy cells and damage them genetically. These active oxygen radicals steal electrons from normal, healthy biological molecules. This electron theft by active oxygen oxidizes tissue and can cause disease.

Because active oxygen can damage normal tissue, it is essential to scavenge this active oxygen from the body before it can cause disintegration of healthy tissue. If we can find an effective method to block the oxidation of healthy tissue by active oxygen, then we can attempt to prevent disease.

![]()

![]()
![]()
![]()

Antioxidants block dangerous oxidation
One way to protect healthy tissue from the ravages of oxidation caused by active oxygen is to provide free electrons to active oxygen radicals, thus neutralizing their high oxidation potential and preventing them from reacting with healthy tissue.
Research on the link between diet and cancer is far from complete, but some evidence indicates that what we eat may affect our susceptibility to cancer. Some foods seem to help defend against cancer, others appear to promote it.
Much of the damage caused by carcinogenic substances in food may come about because of an oxidation reaction in the cell. In this process, an oddball oxygen molecule may damage the genetic code of the cell. Some researchers believe that substances that prevent oxidation -- called ANTIOXIDANTS -- can block the damage. This leads naturally to the theory that the intake of natural antioxidants could be an important aspect of the body's defense against cancer. Substances that some believe inhibit cancer include vitamin C, vitamin E, beta-carotene, selenium, and gluthione (an amino acid). These substances are reducing agents. They supply electrons to free radicals and block the interaction of the free radical with normal tissue.
As we mentioned earlier, the presence of toxic waste products such as hydrogen sulfide, ammonia, histamines, indoles, phenols and scatoles impart an offensive odor to human feces. In the medical profession, it is well known that patients suffering from hepatitis and cirrhosis pass particularly odoriferous stools.
Excessively offensive stools caused by the presence of toxins are indicators of certain diseases, and the body responds to the presence of these toxins by producing neutrophil leukocytes to release active oxygen in an attempt to neutralize the damage to organs that can be caused by such waste products. But when an excess amount of such active oxygen is produced, it can damage healthy cells as well as neutralize toxins. This leads us to the conclusion that we can minimize the harmful effect of these active oxygen radicals by reducing them with an ample supply of electrons.